- Effects on Phase Behavior
- Enhancing Mobility
- Phase Selective Chemistry
A fluid, or any substance, can be in a supercritical state above its critical temperature (Tc) and critical pressure (Pc). Tc and Pc denote the highest temperature and pressure at which the fluid co-exists as liquid and vapor in equilibrium. The distinguishing characteristics of supercritical fluids (SCF) are adjustable solvent strength and gas-like transport properties. The following table shows a comparison between liquid and SCFs.
| Diffusivity (cm2/sec) | Viscosity (cps) | Density (g/cm3) | Surface Tension (dynes/cm) | |
| SCF | 10-3 | 0.02 – 0.05 | 0.2 – 1.0 | 0 |
| Liquid | 10-5 | 1 | 0.8 | 20 - 50 |
We use supercritical carbon dioxide (SC CO2) for most of our study, because of its moderate critical properties (Tc = 31 °C; Pc = 73.8 bar). Most polymers are insoluble in SC CO2, excluding amorphous fluoropolymers, polysiloxanes and some polycarbonate copolymers. Though most of the polymers fail to dissolve, they can be swollen in SC CO2. Sorption of CO2 has significant effect on the properties of the polymer. The most important effects are the depression of Tg, the enhancement of small molecules and polymer chain diffusivity within dilated or swollen polymers, and an increase in the free volume present in the polymer. Research being done in our group involves exploring and utilizing the above-noted effects in various polymeric systems. Our recent work can be broadly divided into the three categories listed below.
Effects of SCF on phase behavior and morphology of block copolymers
The influence of CO2 sorption on the closed-loop phase diagram of Polystyrene-block-poly(n-pentyl methacrylate) (PS-b-PnPMA) copolymers are shown below. Differential dilation of the constituent blocks was used to induce microphase separation of the PS-b-PnPMA. With increasing carbon dioxide loading, an appreciable expansion in the size of the closed-loop was found. At a carbon dioxide activity of 0.1, the upper order-to-disorder transition temperature (UODT) was elevated by more than 20 °C; whereas the lower disorder to-order transition temperature (LDOT) was depressed by the same magnitude. This arises from the entropic nature of the closed-loop miscibility gap in this weakly interacting system, where microphase separation is driven by disparity in free volumes between dissimilar segments of the chain.
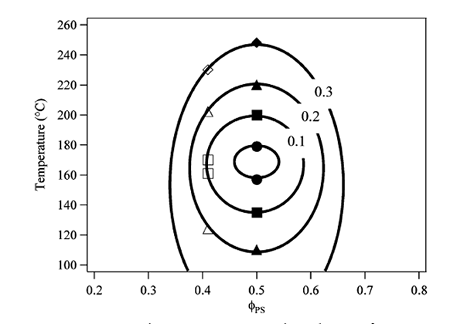
Figure 1: UODT and LDOT temperatures in 50 kDa PS-b-PnPMA at CO2 activities of 0.0 (circles), 0.1 (squares), 0.2 (triangles), and 0.3 (diamonds) for volume fraction ratios of 50:50 (closed symbols) and 42:58 (open symbols). |
Effect of enhanced mobility on morphology of novel block copolymers
Using a series of AnBn miktoarm star block copolymers with different numbers of arms (n = 1, 2, 4, and 16), the effect of molecular architecture on the grain growth kinetics was investigated by annealing in supercritical carbon dioxide (Figure 2). The grain growth kinetics was monitored in real space by transmission electron microscopy (TEM), followed by micrograph image analysis. It was found that the molecular architecture significantly influenced the grain growth kinetics of these AnBn star block copolymers. The grain growth kinetics for the AnBn stars with n = 2, 4, and 16 were found to be similar for both supercritical CO2 and thermal annealing. However, the grain growth kinetics of the diblock (AnBn, with n = 1) were, relative to thermal annealing, dramatically enhanced in supercritical CO2 .
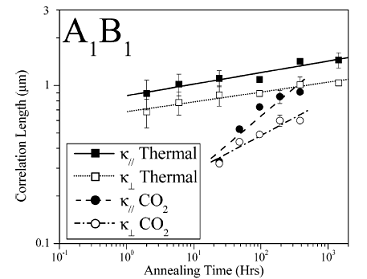 Figure 2: Comparison of the growth of correlation lengths in the A1B1 star block copolymers between supercritical CO2 annealing and thermal annealing. The lines in the plot are the least-squares, power law fits to the corresponding correlation length. Figure 2: Comparison of the growth of correlation lengths in the A1B1 star block copolymers between supercritical CO2 annealing and thermal annealing. The lines in the plot are the least-squares, power law fits to the corresponding correlation length. |
(iii) Phase selective chemistry in block copolymer domains:
Enhanced diffusivity of small molecules in CO2- swollen polymer matrices can be used advantageously to deliver reactants into the block copolymer domains without disturbing the microphase separated nanostructures. Specific affinity between the reactants and one of the block copolymer domains can be manipulated to carry out phase selective reactions in block copolymer domains. Figure 3 shows the cross-section of the nanocomposite film having silver particles deposited selectively in PVP domains of the PS-b-PVP block copolymer film3. Figure 4 shows AFM image of the patterned mesoporous silicate structures obtained by performing domain selective condensation of precursors within self-assembled block copolymer templates by using supercritical CO2 as a delivery medium4. The domain selectivity is imparted by the segregation of acid catalyst into hydrophilic domains. Further, by using a photo acid generator, the presence of acid within the film can be controlled spatially via photolithography. Thus patterns at two different length scales i.e., at nanoscale from self-assembled block copolymer and microscale from photolithography can be generated simultaneously.
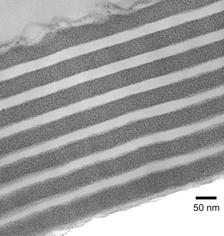 Figure 3: TEM cross-sectional image of the PS-b-P2VP (54K-b-50K) film metallized with Silver particles in P2VP domains. Silver particles are the result of reduction of an organometallic precursor(Ag(COD)hfac). Figure 3: TEM cross-sectional image of the PS-b-P2VP (54K-b-50K) film metallized with Silver particles in P2VP domains. Silver particles are the result of reduction of an organometallic precursor(Ag(COD)hfac). |
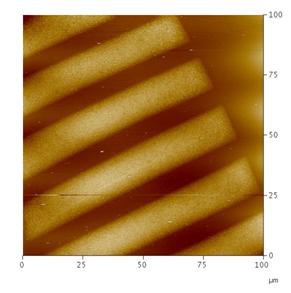 Figure 4: AFM image of the patterned mesoporous silicate structures templated from chemically amplified block copolymer films. Silicate network was obtained by hydrolysis and condensation of silicate precursor(Tetra ethyl orthosilicate) Figure 4: AFM image of the patterned mesoporous silicate structures templated from chemically amplified block copolymer films. Silicate network was obtained by hydrolysis and condensation of silicate precursor(Tetra ethyl orthosilicate) |
