 Distinguished Professor Phone: 413-577-1512 Email: tmccarthy@polysci.umass.edu Meet the McCarthy Group! | Degree Information:B.S. Chemistry, University of Massachusetts Amherst, 1978Ph.D. Organic Chemistry, Mass. Institute of Technology, 1982 Mailing Address:Department of Polymer Science and EngineeringRoom: A512, Conte Research Center University of Massachusetts Amherst 120 Governors Drive Amherst, MA 01003 |
Research InterestsSilicones, Reactive polymer networks, Wetting – contact lines and capillary bridges, Covalently attached monolayers; Polymer and inorganic surface modificationCurrent ResearchTwo Recent Research Highlights:
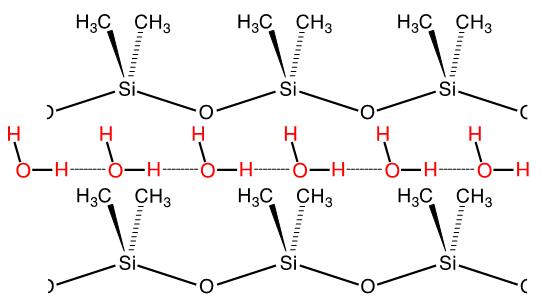
1. It is well known (commercial products are numerous) that silicones (crosslinked polydi-methylsiloxane, PDMS) are extremely water-repellent, in fact they are documented to be the most hydrophobic materials. What is not as well known is that these materials are highly permeable to water vapor. We recently proposed an explanation for this anomalous behavior by measuring vapor permeation rates through a series of silicone films of variable thickness. Vapor transmission rate increases, as expected when membrane thickness is decreased from 160 to 15 mm, but does not increase further below this value. Rate-limiting sorption (adsorption and dissolution) is implicated as the cause of this effect and substantiated by surface modification of the silicone membranes to enhance adsorption rate. A mechanism is proposed that involves one dimensional condensation (to form a linear “polymer” of hydrogen bonded water molecules) in dynamic hydrophobic nanopores.
| |
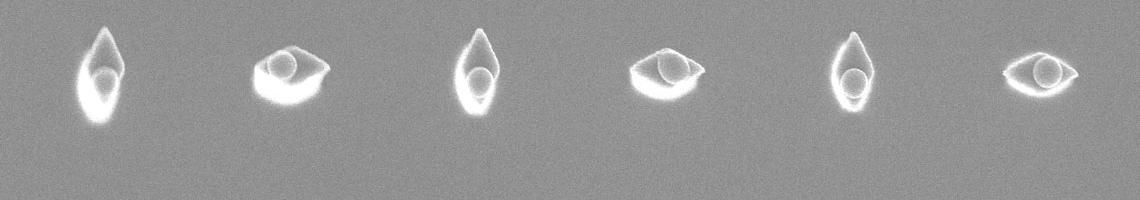
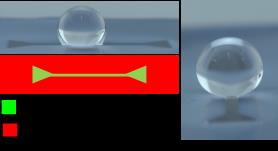 2. We recently prepared superhydrophobic surfaces containing guiding lines that control water motion. The background surfaces exhibit contact angles of qA/qR = 173°/171° (extremely water repellant) and the guiding lines also exhibit hydrophobic behavior (qA/qR = 104°/102°). The low contact angle hysteresis allows facile water motion. Patterns of lines that can be drawn by a combination of photolithography and surface chemistry permit microfluidic manipulation of droplets. The sequence of steps used to prepare these surfaces is central to their success, was designed to minimize defects, and involves only two inexpensive and fluorine-free reagents: methyltrichlorosilane and dimethyldichloro-silane. The disparity in receding contact angles was identified as the key parameter for guided motion.
2. We recently prepared superhydrophobic surfaces containing guiding lines that control water motion. The background surfaces exhibit contact angles of qA/qR = 173°/171° (extremely water repellant) and the guiding lines also exhibit hydrophobic behavior (qA/qR = 104°/102°). The low contact angle hysteresis allows facile water motion. Patterns of lines that can be drawn by a combination of photolithography and surface chemistry permit microfluidic manipulation of droplets. The sequence of steps used to prepare these surfaces is central to their success, was designed to minimize defects, and involves only two inexpensive and fluorine-free reagents: methyltrichlorosilane and dimethyldichloro-silane. The disparity in receding contact angles was identified as the key parameter for guided motion.